Research Project Details
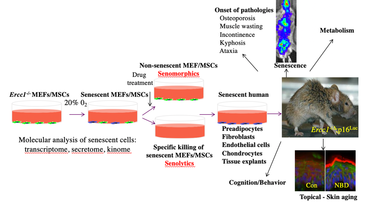
Development of senotherapeutics: Therapeutic approaches to kill specifically senescent cells (senolytics) or suppress senescence including SASP (senomorphics) have the potential to extend healthspan and lifespan. Using both bioinformatics and senescent cell screening approaches, we recently identified several pro-survival pathways, including the BCL-2/BCL-XL, p53/p21, PI3K/AKT, and serpine anti-apoptotic pathways that, when inhibited, result in death of senescent murine and human cells. A combination of the drugs, dasatinib and quercetin (D+Q), which target several of these pro-survival pathways, induces death specifically in senescent murine and human cells in vitro as well as enhances cardiovascular function in aged mice, treadmill endurance in radiation-exposed mice, and decreases frailty, neurologic dysfunction and bone loss in the Ercc1-/∆ progeroid mouse model of accelerated aging. Furthermore, treatment with D+Q reduced senescent cell burden and aortic calcification in the aortae of atherosclerotic ApoE-/- mice improved lung function in a mouse model of idiopathic pulmonary fibrosis, reduced hepatic steatosis prevented age-related bone loss, reduced brain pathology in a mouse model of Alzheimer’s Disease and extended lifespan in naturally aged mice. We also demonstrated that the natural compounds fisetin, a quercetin-related flavonoid, has senolytic/senomorphic activity in certain cell types in culture with fisetin treatment extending healthspan in both progeroid and naturally aged mice and reducing senescence in aged non-human primates. Finally, using a cell-based screening assay, we identified the HSP90 inhibitors 17-DMAG and geldanamycin as potent senolytics, able to improve healthspan in the Ercc1-/∆ mouse model of accelerated aging. We currently are screening for novel senolytics and senolytic combinations using the cell culture assay depicted.
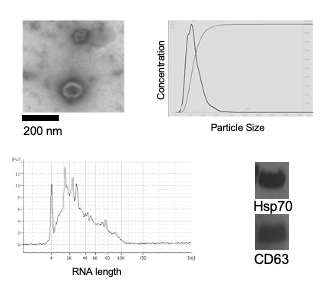
Stem cells and extracellular vesicles: A universal characteristic of aging is loss of tissue regenerative potential, which leads to an impaired ability to respond to stress and, as a consequence, a dramatic increase in the risk of morbidity and mortality. This and the exponentially increased incidence of numerous degenerative diseases in the elderly has led to the hypothesis that aging is caused, in part, by the loss of functional stem cells necessary for maintaining tissue homeostasis. However, what drives age-associated stem cell loss and dysfunction, and the effects on aging have not been clearly defined. It also remains unclear if the defects in stem cell function arise due to defects in the stem cells (cell autonomous) or in the stem cell niche (non-autonomous). We have using both naturally aged mice and mouse models of accelerated systemic and tissue specific aging to examine pathways that drive stem cell dysfunction with age as well as the ability of transplantation of functional stem cells to extend healthspan. Our results demonstrate that injection of young, but not old stem cells extends healthspan and lifespan, consistent with loss of stem cell function contributing to driving aging. In addition, we have identified extracellular vesicles (EVs) secreted by young stem cells as important for conferring the therapeutic effect on aging by suppressing senescence/SASP markers. We currently are developing and optimizing human adult stem cell EVs for clinical application to suppress senescence and extend healthspan.
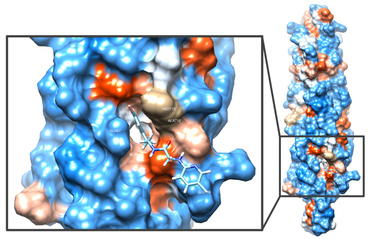
IKK/NF-κB signaling: Persistent DNA damage that accumulates with aging activates a DNA damage response (DDR) that promotes sustained cell-cycle arrest and is required for the senescence-associated secretory phenotype (SASP). Activation of transcription factor NF-κB has been linked to aging pathology and various aging-related diseases, in part, by promoting expression of SASP. DNA damaging agents such as etoposide trigger activation of NF-κB, which requires ATM autophosphorylation and nuclear translocation of the IκB kinase (IKK) regulatory subunit NEMO/IKKß. However, it is still poorly characterized whether the accumulation of DNA damage with aging drives the SASP phenotype through ATM/NEMO-mediated NF-κB activation. We are using the Ercc1-/Δ mouse model of accelerated aging that spontaneously develops numerous age-related conditions including osteoporosis, sarcopenia, intervertebral disc degeneration, glomerulonephropathy, peripheral neuropathy and loss of cognition, to address the causal link between persistent DNA damage, ATM, NF-κB activation and aging. We have generated and are characterizing Ercc1-/∆ mice heterozygous for NF-κB /p65, ATM, TNF-R, MyD88 or a NEMO mutation that prevent activation by NF-κB . We also are using a novel small molecule inhibitor (SR12343) of IKK that we recently developed to block activation of IKK/NF-κB to extend healthspan in mouse models of aging. SR12343 blocks the interaction between IKKß and NEMO (see figure).
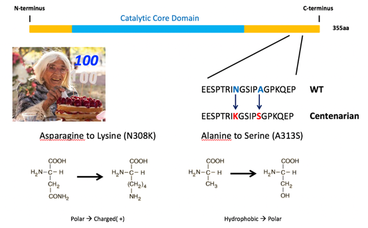
Identifying longevity-associated rare variants in centenarians to guide drug development: Traditionally, drug development is geared towards specific diseases, with increasing attention to genetic variation in the human population as a basis for drug target identification and validation. A genetic approach is highly applicable to the development of drug targets for promoting longevity and healthy aging in view of the discovery that human lifespan has a genetic component, which is stronger in individuals reaching extreme old age, i.e., centenarians. In collaboration with investigators at Albert Einstein School of Medicine, we have used DNA sequence analysis of large, well-defined human cohorts of nonagenarians, centenarians, and super centenarians (those living to 110) to identify rare variants associated with longevity. This has led to identification of coding and non-coding variants in IKK/NF-κB family members, Smad3, SIRT6, FOXO3a, IGF-1R and ATM. Using this information, the lab is identifying compounds that mimic the biological effect of the variants. In particular, we are focusing on developing compounds that mimic the effects of the two amino acid variants in SIRT6 that improve DNA repair activity of SIRT6.